Recently, Qing Jiang and his team at Nanjing University identified likely pathogenic variants in the LRP1 (low-density lipoprotein receptor-related protein 1) gene in two families and seven unrelated Developmental dysplasia of the hip (DDH) patients (Fig. 1). The timing of triradiate cartilage development was brought forward 1 or 2 weeks earlier in the LRP-deficient mice, which leads to malformation of the acetabulum and femoral head. Moreover, Lrp1 deficiency caused a significant decrease of chondrogenic ability in vitro. The results have been published on September 6, 2022 in The Proceedings of the National Academy of Sciences (PNAS) entitled “Heterozygous LRP1 deficiency causes developmental dysplasia of the hip by impairing triradiate chondrocytes differentiation due to inhibition of autophagy” (DOI: 10.1073/pnas.2203557119).
The paper received high evaluations from two reviewers: “It is an interesting observation of the relationship between LRP1 and its protective effect on DDH in humans and in mice.”; “The paper reports interesting findings and reveal a novel function of Lrp1.” Prof. Jasper Rine, the PNAS editorial member, also commented that “The discovery of LRP1 mutations in 2 families with DDH and its validation in a knockin mouse model is significant and warrants in principle publication in PNAS.”

Fig. 1.A schema of the proposed molecular mechanism in controlling acetabular development.
Developmental dysplasia of the hip (DDH) is one of the most common congenital skeletal malformations. Anatomically, DDH is defined as the mismatch of the femoral head and acetabulum. The acetabula of DDH patients show a significantly decreased volumes compared to the normal acetabula. The decrease of acetabular volumes increases local stress on the articular surface and the instability of the hip joint, causing pain and disability of the hip joint and eventually OA. Development of an acetabulum depends on the hemispherical acetabular cartilage and the Y-shaped triradiate cartilage. The acetabular cartilage deepens the acetabulum due to interstitial growth and peripheral deposition. The triradiate cartilage increases the height and width of the acetabulum by bidirectional growth, resulting in increases of acetabular surface area and volume. However, the etiology of DDH and the molecular mechanism controlling acetabular development remains unclear.
Faced with the above challenges, Yan et al. conducted whole-exome sequencing in eight DDH families followed by targeted sequencing of 68 sporadic DDH patients. All patients harboring the LRP1 variants presented a typical DDH phenotype. A knockin (KI) mouse of an Lrp1 missense variant corresponding to a human variant identified in DDH and an Lrp1 knockout (KO) mouse mimicked human DDH phenotypes. A critical role of LRP1 in the development of the Y-shaped triradiate cartilage was discovered by using the DDH model mice with Lrp1 deficiency. The heterozygous Lrp1 knockout (KO) mouse (Lrp1+/−) showed phenotypes recapitulating the human DDH phenotypes, indicating Lrp1 loss of function causes DDH. Lrp1 knockin mice with a missense variant corresponding to a human variant identified in DDH (Lrp1R1783W) also presented DDH phenotypes, which were milder in heterozygotes and severer in homozygotes than those of the Lrp1 KO mouse (Fig. 2).
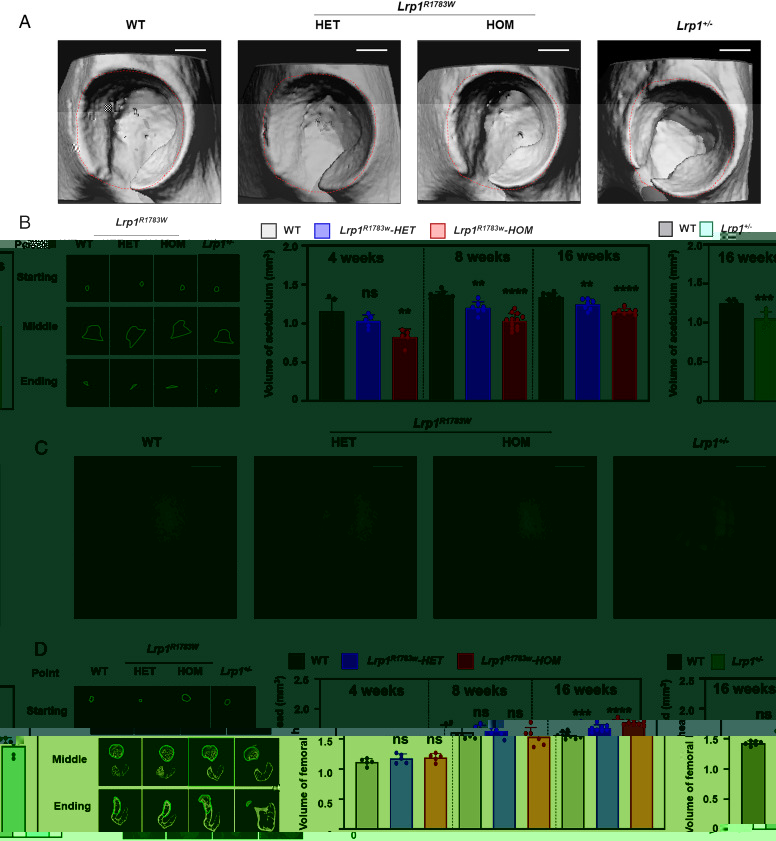
Fig. 2. 3D micro-CT images of the DDH phenotype of heterozygous and homozygous Lrp1R1783W mice, Lrp1+/- mice, and their WT littermates at 8 wk. (A) Micro-CT images of the acetabulum. (B) The volume of the acetabulum measured according to the ROI. (C) Micro-CT images of the femoral head. (D) The volume of the femoral head. Volumes were measured according to the ROI. Scale bar: 1 mm. Values = means ± SD; ns, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001; HET, heterozygote; HOM, homozygote.
The timing of triradiate cartilage development was brought forward 1 or 2 weeks earlier in the LRP-deficient mice, which leads to malformation of the acetabulum and femoral head (Fig. 3). To further characterize the LRP1 function at the cell level, BMSCs were isolated from WT and heterozygous KO mice and their chondrogenic potential was tested. Lrp1 deficiency caused a significant decrease of chondrogenic ability in vitro. During the chondrogenic induction of mice bone marrow stem cells and ATDC5 (an inducible chondrogenic cell line), Lrp1 deficiency caused decreased autophagy levels with significant β-catenin up-regulation and suppression of chondrocyte marker genes. The expression of chondrocyte markers was rescued by PNU-74654 (a β-catenin antagonist) in an shRNA-Lrp1–expressed ATDC5 cell. Our study reveals a critical role of LRP1 in the etiology and pathogenesis of DDH, opening an avenue for its treatment.

Fig. 3. Histology of the hip joints of heterozygous and homozygous Lrp1R1783W mice, Lrp1+/- mice, and their WT littermates. (A) Sections of mice acetabulum cartilage at 40x magnification at 8 wk. Scale bar: 100 μm. Lrp1R1783W and Lrp1+/- mice showed sparser acetabulum cartilage compared with WT mice. (B) The unmineralized cartilage area calculated using the contrast red-green feature. Values = means ± SD; *P < 0.01; **P < 0.0001. The area of WT mice was significantly larger than those of Lrp1R1783W and Lrp1+/- mice. (C) Sections in 10x magnification at 4, 6, 8, and 16 wk. Scale bar: 200 μm. The triradiate cartilage had closed completely before 6 wk in KI and KO mice, while it closed after 8 wk in WT mice. The quantitative results of maximum cross-sectional of the actabulum area (D) and maximum cross-sectional of the acetabulum cartilage area (E) was shown. (F) Schematic of the LRP1-deficient hip joint. Premature fusion of the triradiate cartilage (red) causes dysplasia of the hip.
Prof. Qing Jiang’s study reveals a critical role of LRP1 in the etiology and pathogenesis of DDH, opening an avenue for its treatment. This work was supported by the National Natural Science Foundation of China and the Central University Basic Research Fund of China.
Link to the paper: Heterozygous LRP1 deficiency causes developmental dysplasia of the hip by impairing triradiate chondrocytes differentiation due to inhibition of autophagy | PNAS



